Abstract
Introduction. Deletion of the short arm of chromosome 17 (del(17p)) is a well-established high-risk feature in multiple myeloma (MM) and is included in current disease staging criteria. Treatment of del(17p) MM is a major challenge, since the disease is characterized by rapid development of chemoresistance and short survival. The size of the del(17p) clone correlates with prognosis, and the threshold value of 55% to 60% has the worst prognosis. Approximately a third of patients (pts) have a concomitant TP53 mutation, with a complete abolition of the protein function. TP53 biallelic inactivation is defined as double-hit myeloma.
To date, very few studies specifically addressed the issue of tailored treatments for del(17p) MM. The IFM 2010-02 trial has shown that the combination of pomalidomide plus dexamethasone is promising in this setting (Leleu et al, Blood 2014). Immunotherapy, and its mechanism of killing, which is independent from TP53-mediated DNA repair, is an attractive strategy for del(17p) MM treatment. We hypothesized that the combination of daratumumab plus pomalidomide plus dexamethasone (DPd) could be effective in the setting of relapsed MM pts harboring a del(17p) clone. Here we report an analysis of the phase II DEDALO trial (NCT04124497).
Methods. Key eligibility criteria included: relapsed or relapsed/refractory disease; up to 3 prior lines of therapy, del(17p) observed by fluorescence in situ hybridization (FISH) in at least 10% of plasma cells at any time of MM history, previous exposure to lenalidomide (Len), absence of refractoriness or intolerance to pomalidomide, and previous exposure to an anti-CD38 monoclonal antibody. Pts received continuous treatment with daratumumab (1800 mg subcutaneously or 16 mg/kg intravenously) weekly during cycles 1 and 2, every 2 weeks during cycles 3-6, and every 4 weeks thereafter; oral pomalidomide (4 mg, once daily on days 1-21); and oral or intravenous dexamethasone (40 mg once daily on days 1, 8, 15, and 22; 20 mg for those aged 75 years or older) at each 28-day cycle. The primary endpoint was minimal residual disease at 10-5 (MRD 10-5) negativity within the first 12 months of treatment. Next-generation flow (NGF) and next-generation sequencing (NGS) MRD analyses were performed. The key secondary endpoints were progression-free survival (PFS), overall response rate (ORR), and overall survival (OS).
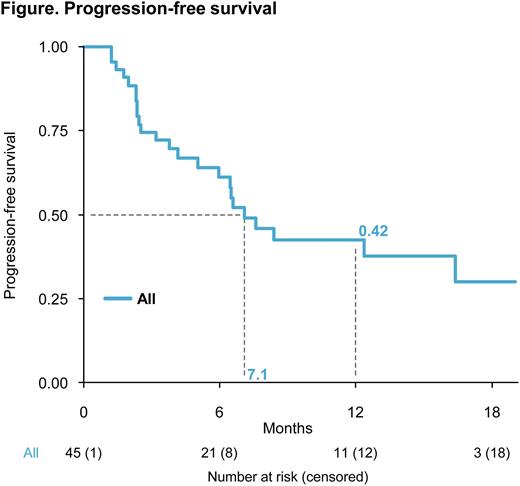
Results. Forty-five pts were enrolled. The median age was 63 (range 43-83) years (yrs), and 60%/29%/11% of pts had International Staging System (ISS) stage I/II/III. All pts had >10% del(17p) and 14 pts had ≥55% del(17p); t(4;14) was observed in 6, t(14;16) in 6, del(1p) in 10, and 1q+ in 16 pts. Median number of prior lines of therapy was 1 (range 1-3); 100% had been previously exposed to Len and 86% to a proteasome inhibitor (PI). Three pts achieved MRD negativity by NGF, while NGS analysis is ongoing. ORR was 60%, with a partial response (PR) in 13, a very good partial response (VGPR) in 12, and a complete response (CR) or better in 2 pts. Median time-to-response was 2.5 months. With a median follow-up of 8.5 months (range 6.3-13.9), median PFS was 7.1 months (range 5.9 - not reached [NR]; Figure). By subgroup analysis, PFS was: 8.4 months in pts with del(17p) clone size <60% and 6.5 months in pts with del(17p) clone size ≥60% (HR, 0.75; 95% CI 0.33-1.7; P=0.48); 12.4 months in pts with ISS I vs 4.2 months in those with ISS II-III (HR, 2.48; 95% CI 1.12-5.51; P=0.02); 6.6 months in pts at first relapse vs 7.1 months beyond first relapse (HR 0.95; 95% CI 0.42-2.12; P=0.89); 7.1 months in pts <65 yrs and 7.6 months in those ≥65 yrs of age (HR 0.84; 95% CI 0.37-1.92; P=0.68). Median OS was NR. No new safety concerns were observed. TP53 mutational analysis is ongoing.
Conclusion. In this difficult-to-treat population, DPd is a therapeutic option for pts of all ages and can be considered as a bridge to other immunotherapies.
Disclosures
Montefusco:Bristol Myers Squibb/Celgene: Membership on an entity's Board of Directors or advisory committees, Other: Speaking fees and travel grants, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Other: Speaking fees and travel grants, Research Funding. Patriarca:Celgene: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees. Mina:Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy. D'Agostino:Sanofi: Membership on an entity's Board of Directors or advisory committees, Other: Honoraria for lectures; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees, Other: Honoraria for lectures. Petrucci:GlaxoSmithKline: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings and/or travel; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings and/or travel; Celgene/Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings and/or travel; Janssen-Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings and/or travel; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings and/or travel; Roche: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees. Paris:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Belotti:Amgen: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees. Cavo:AbbVie, Amgen, Bristol Myers Squibb/Celgene, Pfizer, GlaxoSmithKline, Sanofi, Roche, Takeda: Consultancy, Honoraria; Janssen: Honoraria, Speakers Bureau. Conticello:GlaxoSmithKline: Honoraria; Janssen: Honoraria; Amgen: Honoraria; Takeda: Honoraria; Sanofi: Honoraria; Bristol Myers Squibb: Honoraria. Carlo-Stella:Incyte: Honoraria; AstraZeneca: Honoraria; Merck Sharp & Dohme: Honoraria; Bristol Myers Squibb: Honoraria; Roche: Other: Consultancy/Advisory, Research Funding; Sanofi: Other: Consultancy/Advisory, Research Funding; Janssen Oncology: Honoraria; Novartis: Honoraria; Karyopharm Therapeutics: Other: Consultancy/Advisory; ADC Therapeutics: Honoraria, Other: Consultancy/Advisory, Research Funding; Takeda: Honoraria; Celgene/Bristol Myers Squibb: Other: Consultancy/Advisory; Scenic Biotech: Other: Consultancy/Advisory. Boccadoro:Mundipharma: Research Funding; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria; Bristol Myers Squibb: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Sanofi: Honoraria, Research Funding.
OffLabel Disclosure:
This presentation includes discussion of the off-label use of a drug or drugs for the treatment of multiple myeloma: daratumumab, pomalidomide, dexamethasone, and lenalidomide.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal